Ixoreal is the industry leader in research and clinical trials investigating the effect of Shatavari, with 10+ studies. It collaborates with major academic and research institutes. All of Ixoreal-partnered clinical studies follow the gold standards of substantiation: randomized, double-blind, placebo-controlled designs. The studies have been conducted on healthy population and are published in PubMed-indexed peer reviewed journals. Our research studies focus on the main applications of Shatavari as listed below.
Clinical Studies
Lactation Support
Title: Shatavari (Asparagus racemosus Willd) Root Extract for Postpartum Lactation: A Randomized, Double-Blind, Placebo-Controlled Study.
Study Dose: 300 mg/day
Study Duration: 72 hours
No. of Participants: 113
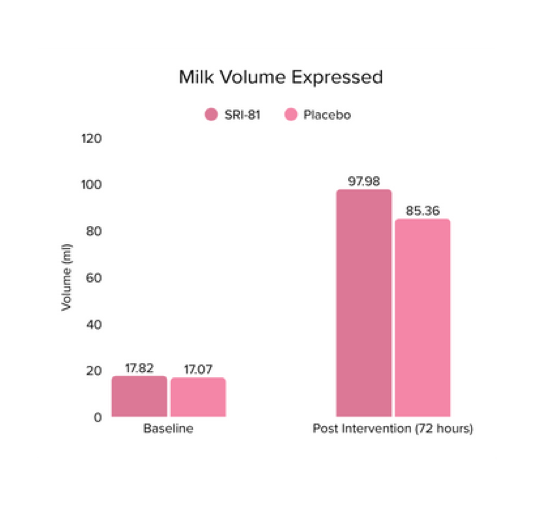
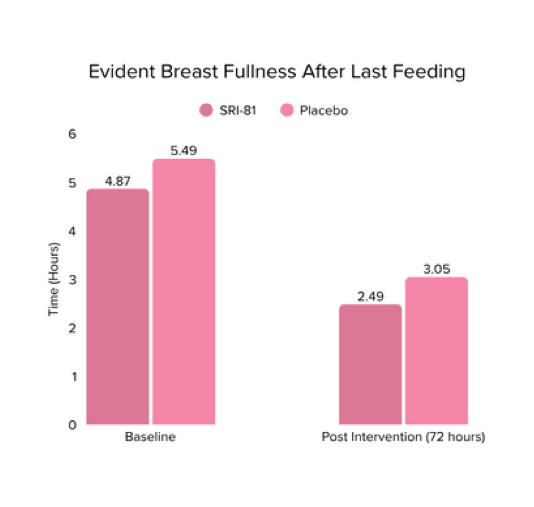
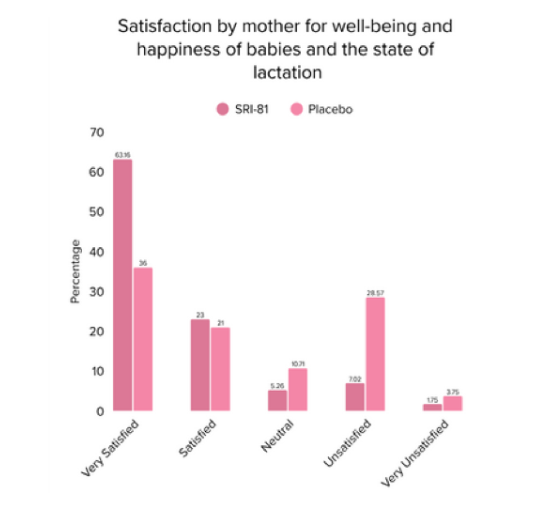
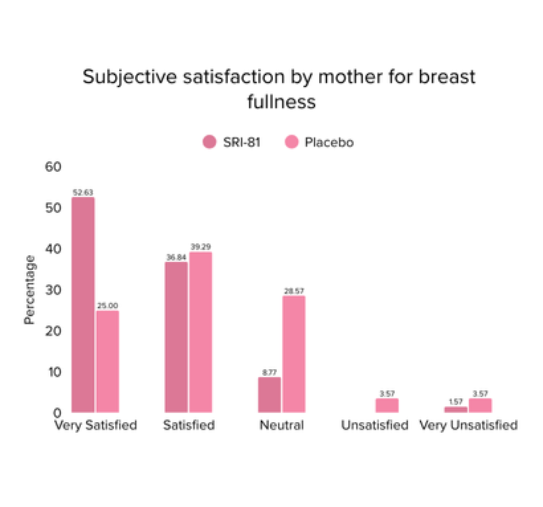
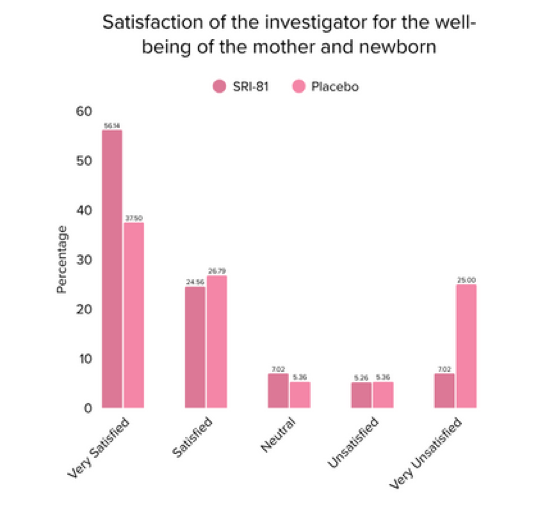
Conclusion: The study demonstrated that Shatavari root extract (300 mg/day) significantly enhanced lactation within 72 hours with a significant decrease in time to noticeable breast fullness. An increase in milk volume and improved maternal satisfaction, as indicated by well-being and happiness scores, was observed. The supplementation was well-tolerated, with no adverse events reported.
Sexual Function in Women
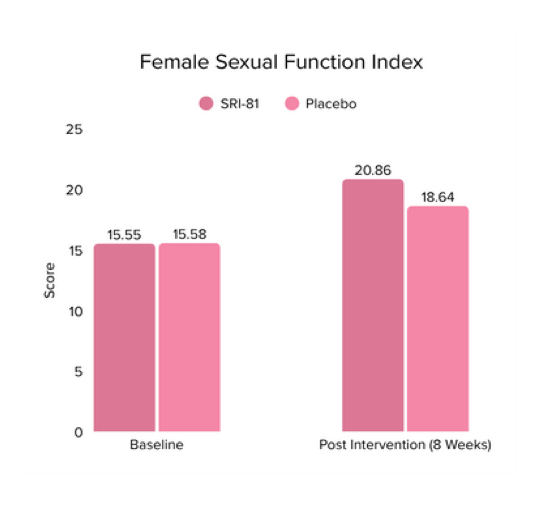
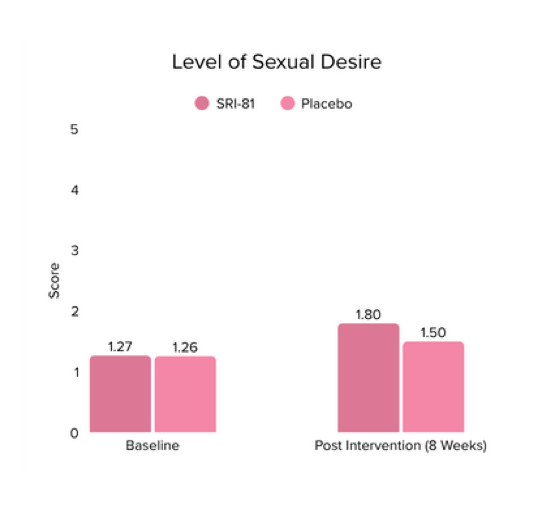
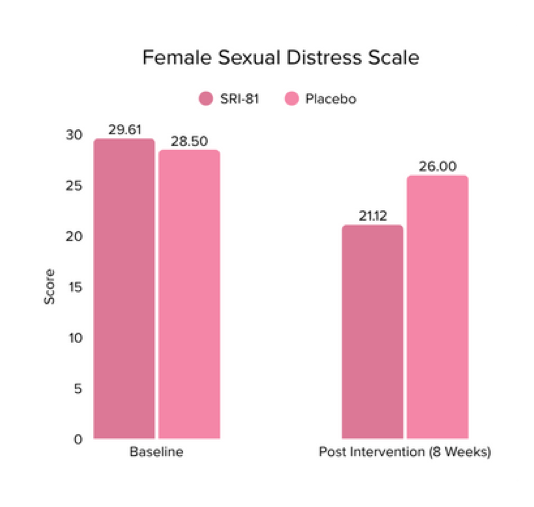
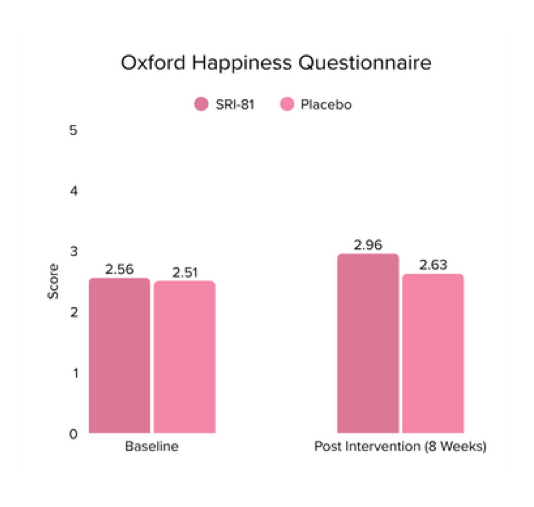
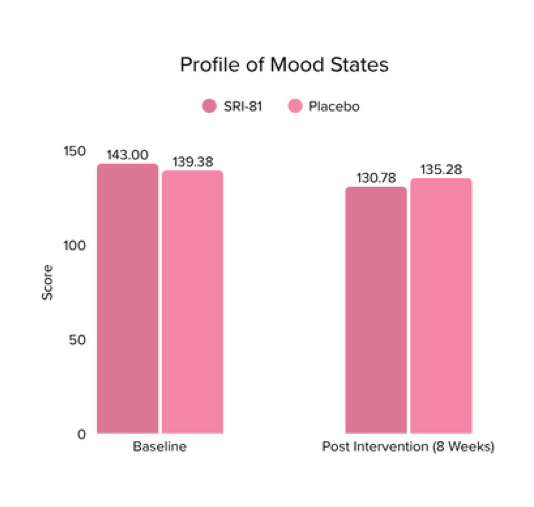
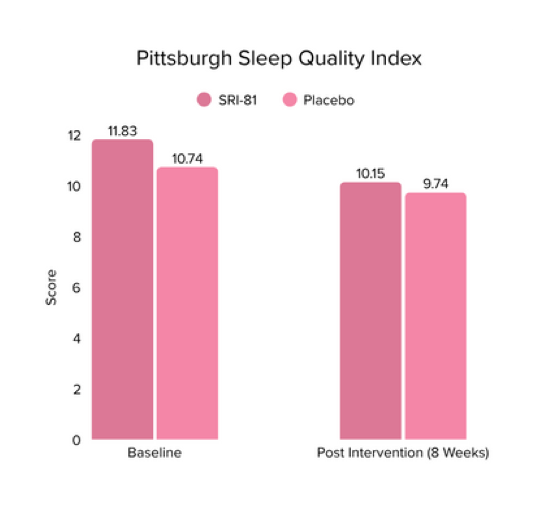
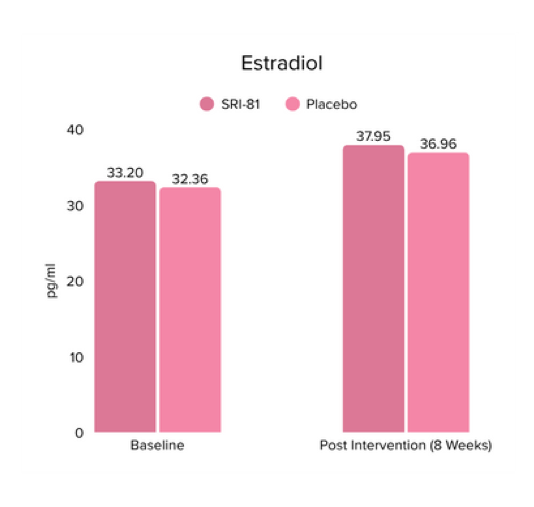
Title: Shatavari (Asparagus racemosus) Root Extract Improves Sexual Wellness in Women: Findings from a Prospective, Randomized, Double-Blind, Three-arm, Parallel-Group, Placebo-Controlled Study.
Study Dose: 300 mg/day
Study Duration: 8 weeks
No. of Participants: 127
Conclusion: The study demonstrated that Shatavari root extract significantly improved sexual wellness, with a 34.2% increase in total Female Sexual Function Index scores and a significant increase in arousal, lubrication, satisfaction, and orgasm. It also reduced sexual distress by 28.7%, improved mood and sleep, and balanced key reproductive hormones, supporting its role in enhancing overall female sexual health.
Managing PCOS in Women
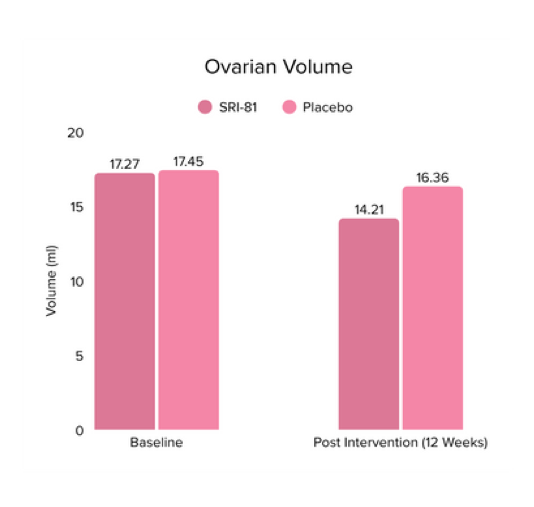
Title: A Randomized, Double-Blind, Two-Arm, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Shatavari in Women with Polycystic Ovarian Disease Syndrome (PCOS).
Study Dose: 300 mg/day
Study Duration: 12 weeks
No. of Participants: 66
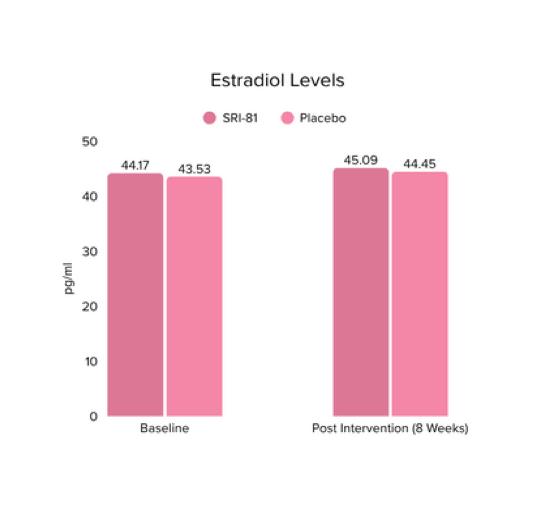
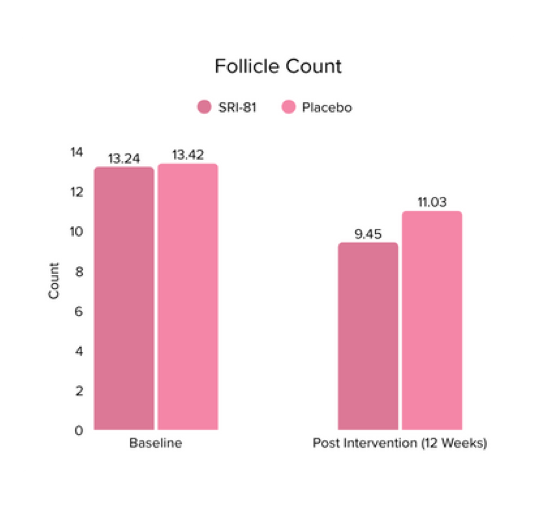
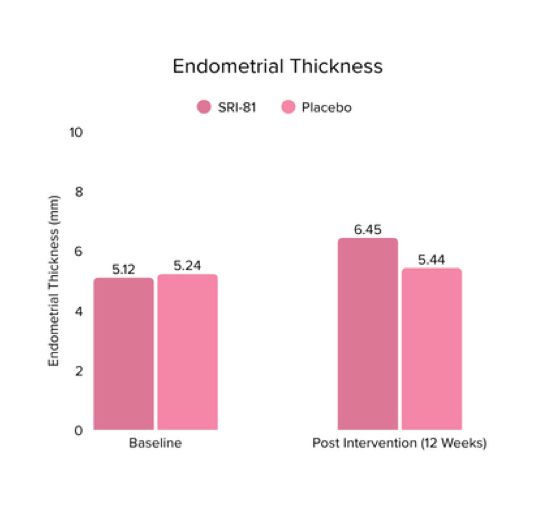
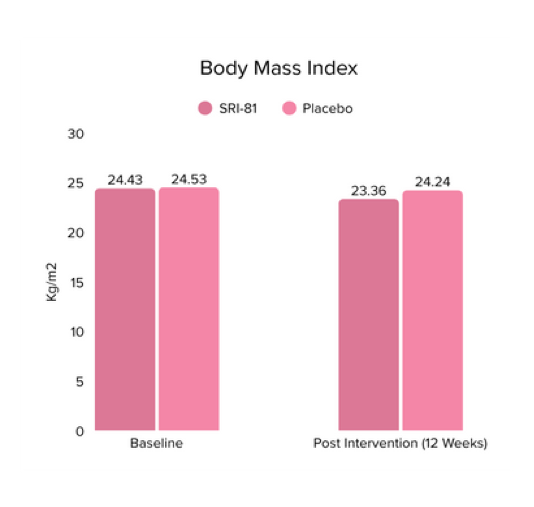
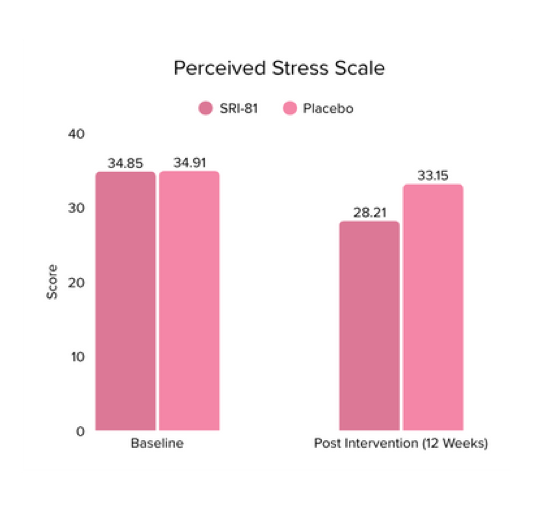
Conclusion: In this study, Shatavari root extract demonstrated significant improvements in hormonal and reproductive parameters. The Perceived Stress Scale (PSS) score decreased by 19.05%. Significant reductions were observed in ovarian volume (17.72%) and follicle count (28.6%), while an increase in endometrial thickness (25.98%) was observed, suggesting potential hormonal-balancing effects. Hormonal markers also showed mild to moderate reductions, including FSH, LH, estradiol, total testosterone, progesterone, and DHEA-S.
Perimenopausal Symptoms in Women
Title: Efficacy and Safety of Shatavari for Treatment of Perimenopausal Symptoms in Women: A Randomized, Double-blind, Two-arm, Parallel, Placebo-controlled Study.
Study Dose: 300 mg/day
Study Duration: 8 weeks
No. of Participants: 73
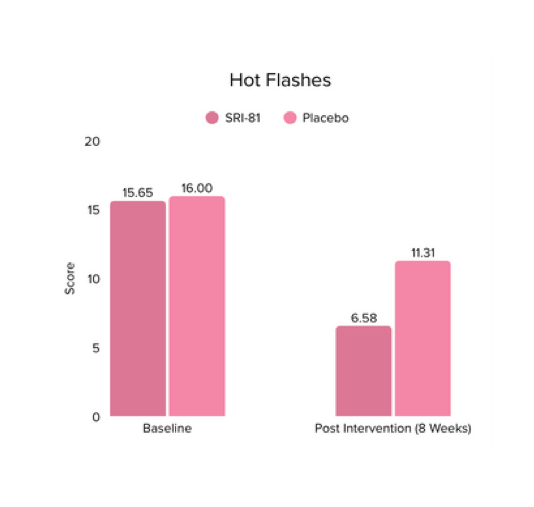
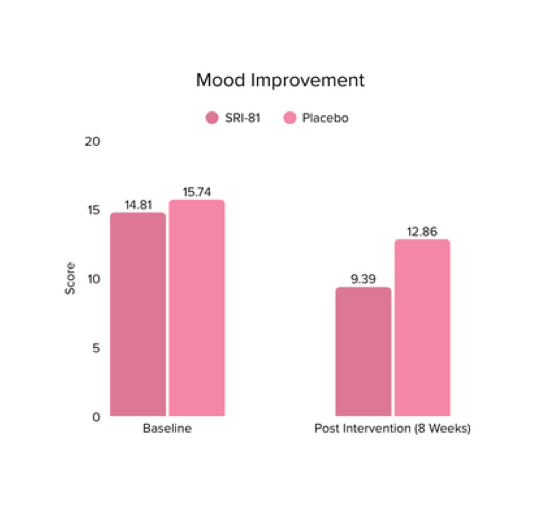
Conclusion: A study in perimenopausal women showed that Shatavari root extract significantly improved climacteric symptoms, with a 42.6% reduction in total Menopause Rating Scale scores and significant improvement across somato-vegetative, psychological, and urogenital domains. Stress scores reduced by 29.6%, hot flashes by 25.9%, and mood disturbances significantly improved. There was also a 32.8% increase in serum estradiol, supporting Shatavari’s role in easing the transition through perimenopause.
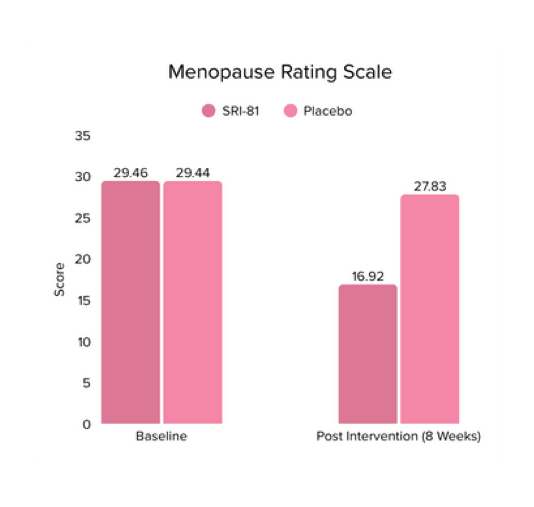
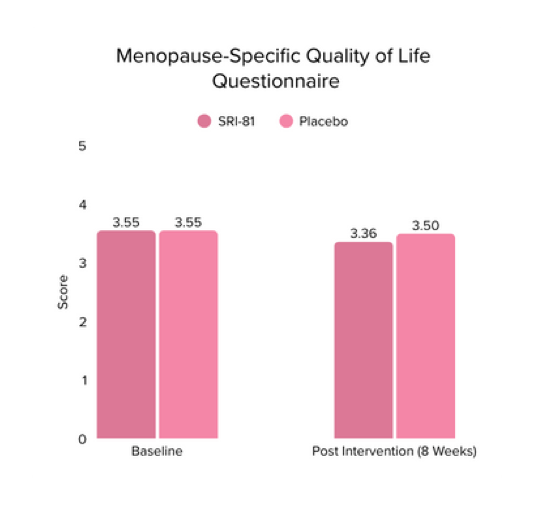
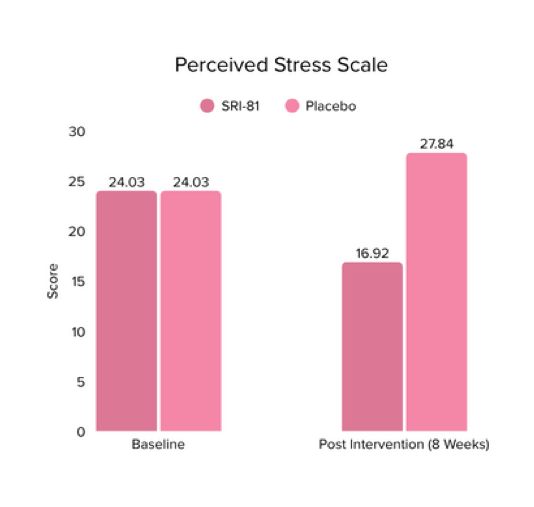
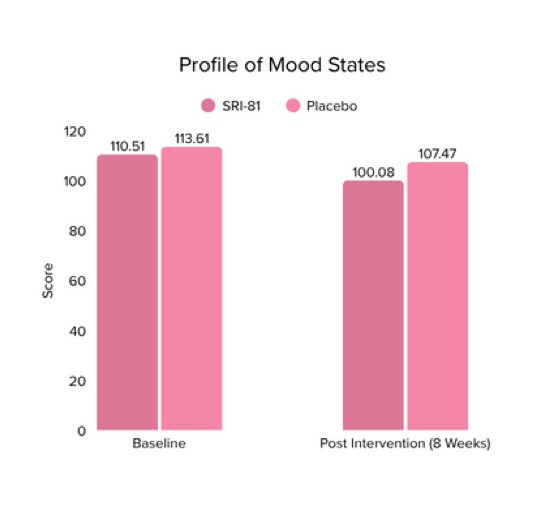
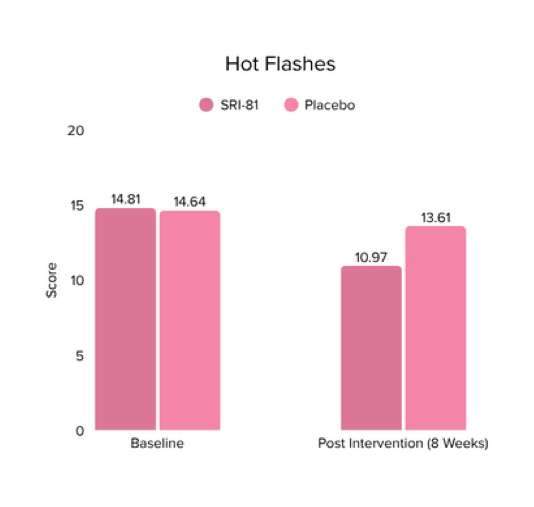
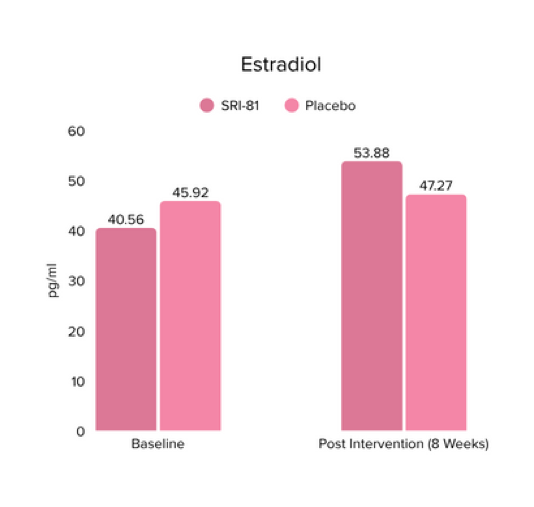
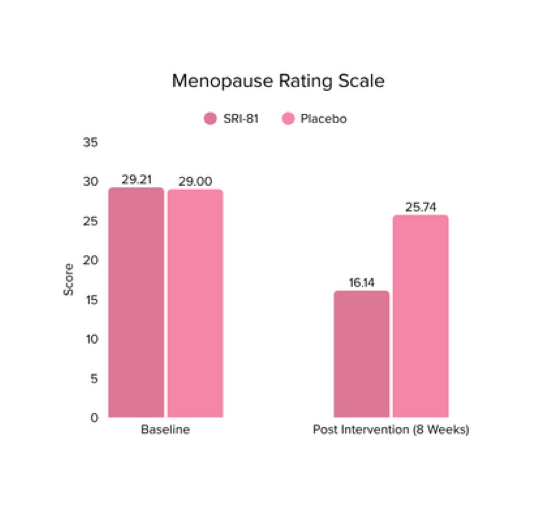
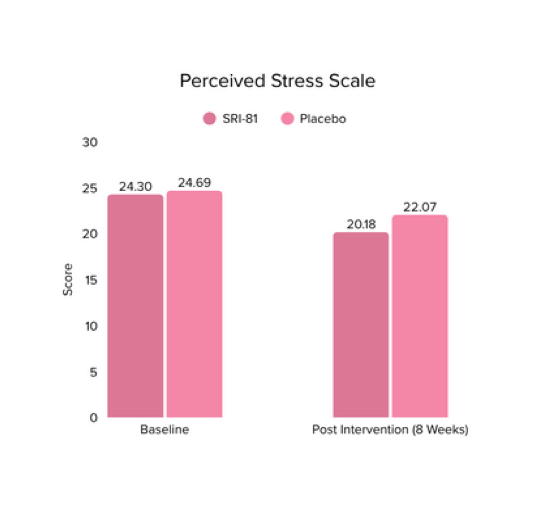
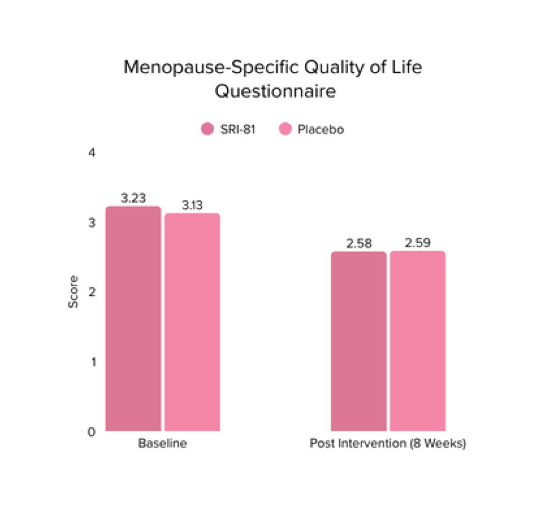
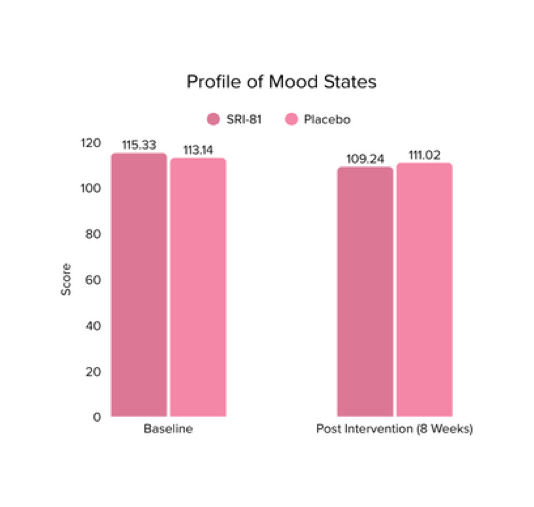
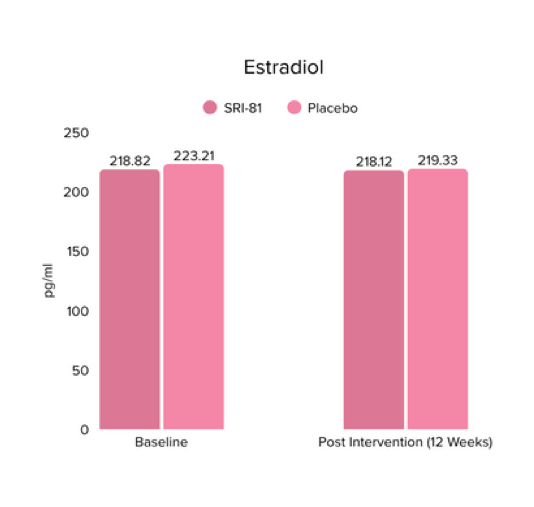
Menopausal Symptoms in Women
Title: Efficacy and Safety of Shatavari Root Extract (Asparagus racemosus) for Menopausal Symptoms: A Randomized, Double-blind, Three-arm, Placebo-controlled Study.
Study Dose: 300 mg/day
Study Duration: 8 weeks
No. of Participants: 125
Conclusion: A study in menopausal women showed that Shatavari root extract significantly improved menopausal symptoms. The total Menopause Rating Scale score significantly decreased by 44.7%, with reductions in somato-vegetative (36.17%), psychological (37.14%), and urogenital (29.81%) domains. Also, a 16.95% reduction in perceived stress and a 57.95% decrease in hot flashes were observed. Quality of life and hormone balance also improved, confirming Shatavari’s efficacy in managing menopause naturally.